1,*Miloš Garabandić and 2Nataša Todorović
1Isidora Sekulić High School, 21000 Novi Sad, Serbia; garabandic.milos@gmail.com
2University of Novi Sad, Faculty of Sciences, Department of Physics, 21000 Novi Sad, Serbia; natasa.todorovic@df.uns.ac.rs
*Corresponding Author
Included in Volume 1, Issue 2 of the AIDASCO Reviews as a Research Article
ABSTRACT: The isotope of hydrogen, tritium, is a radioactive isotope. In most cases, it is found bonded to molecules that replace hydrogen. It can be found in nature as a product of interactions of cosmic radiation with air or atmospheric molecules nitrogen. Such tritium is natural. There is also anthropogenic tritium that contributes to increased levels of natural tritium. Artificially produced tritium is produced in the testing of nuclear weapons, in the operation of nuclear power plants, and processing of nuclear fuel. This paper will describe the methods of tritium detection liquid scintillation detector Quantulus 1220 by the direct method in 300 minutes at the Department of Nuclear Physics. Surface water samples with tritium were taken from the location of PC Nuclear facilities of Serbia from the Mlaka stream. The monitoring recorded increased tritium content at two locations, indicating failures of nuclear waste storage.
Keywords: tritium; radioactivity; detector; quantulus1220
1. Introduction
The concentration of tritium on Earth is very low. The atmosphere only has quantities of traces created by interacting its gases with cosmic rays. The atomic nucleus is unstable and decays with a half-life of 12.32 years per emission of one of the electrons in 3He (beta decay) [1–9]. The general properties of tritium are given in the table.
Table 1. Tritium basic properties [1,10]
| Property | Value |
|---|---|
| Spin | 1/2+ |
| Half-Life period | 12.3 years |
| Atomic mass | 3.01605 u |
| Melting point | -252.5 °C |
| Boiling point | 248.1 °C |
2. Thritium detection technique
- Liquid scintillation counting involves mixing the tritium sample with a scintillation cocktail, emitting light when the tritium decays. The emitted light is then detected and counted using a photomultiplier tube.
- Gas proportional counting: In this technique, the tritium sample is mixed with a proportional gas, and the emitted ionization from tritium decay is measured using a gas-filled detector.
- Ionization chamber: An ionization chamber measures the ionization created by tritium decay in the sample. The chamber consists of a gas-filled container with two electrodes, and the amount of ionization is proportional to the amount of tritium in the sample.
- Electrochemical methods involve using an electrode to detect tritium in a sample. The tritium atoms are ionized at the electrode surface, and the resulting current is measured.
- Scintillation spectrometry: This technique uses a scintillation detector to measure the energy of the emitted light from tritium decay, allowing for the identification and quantification of tritium in a sample [17,18].
In this paper, we used the LSC method so we will base it on it.
2.1. Preparation of the sample
First and foremost, in the LSC method, the radioactive sample (for example, tritium water after combustion) should be combined with the scintillation cocktail so that it is in direct contact with a substance that tends to fluoresce when the atoms are excited, thus creating scintillations, i.e., photons. A substance commonly labeled as “fluorine” proved to be appropriate. Fluorine is an appropriate name because of the substance’s ability to scintillate. It lights up when the atoms are excited, so it does not directly refer to the element fluorine [18–24].
The tritium sample is typically dissolved or suspended in a solvent compatible with the liquid scintillator. Mixing with the scintillator: The sample is then mixed with the liquid scintillator, typically a cocktail consisting of a solvent, a scintillant, and other additives to enhance the counting efficiency and reduce background noise. Counting the emitted light: The sample/scintillator mixture is placed in a counting vial and inserted into the LSC instrument. The instrument uses a PMT to detect and count the emitted light from tritium decay in the sample. Data analysis: The counting data is analyzed to determine the concentration of tritium in the sample, typically reported in units of becquerels per liter (Bql-1) or disintegrations per minute (DPM) (Fig. 1).
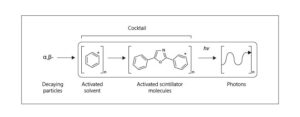
There are several methods of testing tritium using liquid scintillation detectors, and they are:
- ASTM – a standard method for testing tritium
- Electrolytic enrichment
- Direct method
We used the direct method in this work, so more will be said.
2.1.1. Direct method
The direct LSC method is a relatively simple and fast technique for tritium detection. It is often used in environmental monitoring, nuclear power plants, and other applications where tritium may exist.
The direct LSC method offers high sensitivity and can detect tritium at very low levels, typically down to tens of tritium atoms per liter of sample. However, care must be taken to minimize background radiation and to ensure that the liquid scintillator is properly calibrated and free of impurities that could interfere with the counting efficiency.
This method is called direct because it does not require electrolytic enrichment. It is direct determines the tritium values. The method belongs to the rapid tests. It is used as the I method for examining limit values of radionuclides in drinking water. It is also applied when checking water radiation at nuclear reactors, which serves for moderation or cooling [20]. The method uses a scintillation detector that can detect small energy values in which the emission of beta particles that accompanies the decay of tritium falls.
The following equipment requires:
- Liquid scintillation detector(Quantulus1220);
- Bottles of 20 ml (plastic);
- Apparatus for filtration;
- Apparatus for distillation.
3. Results
Before measuring, we define locations and reference values (Fig. 2). Locations:
- Mlaka 1- Place without any impact of nuclear facilities on the environment.
- Mlaka 2- Sampling site at the recipient.
- Mlaka 3-Sampling site downstream from the recipient.
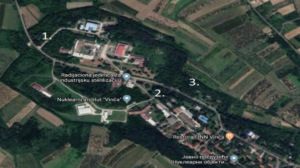
For the detection of tritium in precipitation, sampling with location Zeleno brdo, which is located 7 km from the Nuclear facility, while for the detection of tritium in surface water was taken from Mlaka 1.
3.1. Detector optimization
The detection efficiency is determined by equation 1:
$$\varepsilon_t=\frac{s}{8A_r}$$
where Ar [Bq/l] is activity concentration of 3H solution (equation 2).
$$A=\frac{r-(b+r_q)}{60 \varepsilon_t V}$$
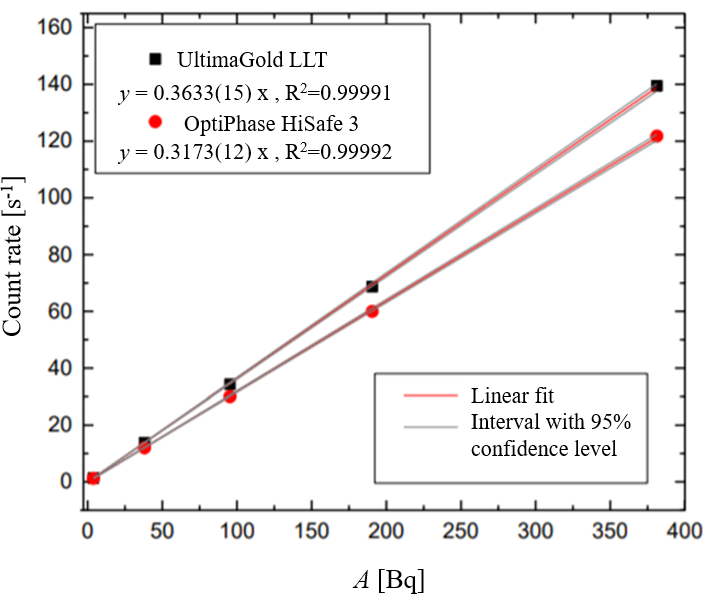
Detection efficiency for Ultima Gold LLT and Optiphase, Ultima Gold LLT is shown in Fig. 3.
The minimum detectable tritium MDA activity achieved during the measurement of 300 min is calculated using equation 3:
$$MDA=\frac{2.71+4.65 \sqrt{(b+r_q)T}}{60 \varepsilon_t V}$$
A display of minimum detectable activity depending on measurement times is shown in Fig. 4.
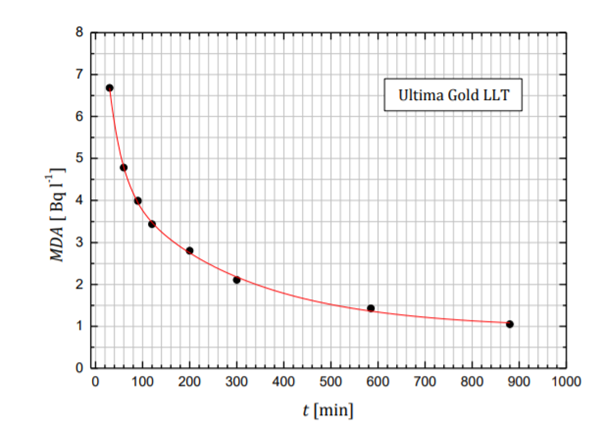
Fig. 5 presents the dependency of specific activity in one year.
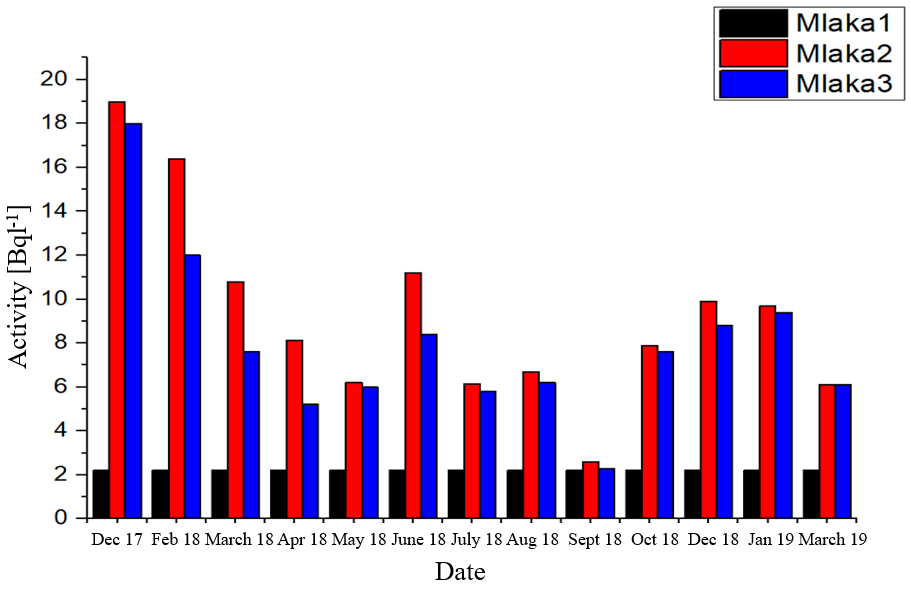
Mean values of tritium concentration for Mlaka 2 and Mlaka 3 in the period from December 2017 to June 2019 are calculated using equation 4:
$$A_{sr}(\sideset{^3}{}{\mathrm{H}})=\frac{A_1+A_2+A_3+…+A_{19}}{19}$$
Values obtained for Mlaka 2: \(A_{sr}(\sideset{^3}{}{\mathrm{H}})_{Mlaka2} = 7.84 \; \mathrm{Bq \; l^-1} \)
Values obtained for Mlaka 3: \(A_{sr}(\sideset{^3}{}{\mathrm{H}})_{Mlaka3} = 6.81 \; \mathrm{Bq \; l^-1} \)
References
1. Special Issue “Conventional and Emerging Extraction Techniques for Compounds from Natural Source and Food”.
Available on-line: https://www.mdpi.com/journal/molecules/special_issues/emerging_extraction#info (accessed on April 18, 2023).
2. Special Issue “Modeling Adsorption Properties of Molecular and Nanostructured Systems for Environmental Applications”.
Available on-line:
https://www.mdpi.com/journal/molecules/special_issues/nanostructured_systems_environmental_applications#info (accessed on April 18, 2023).
3. Hemijska Industrija journal. Available on-line: https://www.ache-pub.org.rs/index.php/HemInd/index (accessed on April 18, 2023).
4. Materials Protection journal. Available on-line: http://idk.org.rs/jmp/ (accessed on April 18, 2023).
5. Journal of Chemists, Technologists and Environmentalists. Available on-line: https://glasnik.tf.unibl.org/ (accessed on April 18, 2023).
6. Journal of Engineering & Processing Management. Available on-line: https://jepm.tfzv.ues.rs.ba/index.php/pub (accessed on April 18, 2023).
7. Book of Abstracts of the 8th International Congress “Engineering, Environment and Materials in Process Industry” – EEM2023, Jahorina, B&H, 20th-23rd March 2023.
