Reshma Tendulkar1,* and Mugdha Tendulkar2
1Vivekanand Education Society’s College of Pharmacy, India; reshma.tendulkar@ves.ac.in
2Sardar Vallabhbhai Patel College of Science, India; mugdhatendulkar14@gmail.com
*Correspondence: reshma.tendulkar@ves.ac.in
– published as website article, to be included in the Volume 1, Issue 1 of the AIDASCO Reviews –
ABSTRACT: Of all malignant glioma forms, glioblastoma is the most frequently present form. The treatment of this highly aggressive tumor form by applying conventional therapies is of low success, leading to a low survival rate. As representatives of immunotherapeutic agents, oncolytic viruses contribute to the revolution in cancer therapy. Although promising, the efficiency of oncolytic viruses for treating glioblastoma is also limited. This is a reason why further optimization of oncolytic virotherapy is necessary. In this work, we review some of glioblastoma’s specificities and provide information about the current status of oncolytic virotherapy.
Keywords: Virotherapy; Oncolytic viruses
1. Introduction
Glioblastoma accounts for 57% of occurrences among all malignant glioma forms and represents the most frequent cause of death when malignant brain tumors are taken into account [1]. This commentary discusses the application of Oncolytic Virotherapy for curing gliomas and highlights various outcomes of the research performed for the practicability of the treatment. The prevalence of glioblastoma accounts for about 0.59 to 5 per 100,000 individuals. The rates continue to rise in many countries. Treating glioblastoma is complex, using chemotherapy, radiotherapy, and debulking surgery. The success rate of surgical debulking is also low, as tumor resection depends upon its associated neurological impairment and location [2]. Recent studies suggest the use of immune checkpoint inhibitors (ICIs). Still, response span and magnitude in solid tumors are found to be fickle. Also, the high molecular heterogeneity of the tumor cells hinders many techniques for curing gliomas. Oncolytic virotherapy is a technique that uses genetically engineered viruses that can preferentially replicate themselves and destroy cancer cells while keeping the surrounding healthy cells unharmed [3]. Oncolytic viruses (OV) kill cancer cells through various mechanisms like apoptosis, pyroptosis, and necroptosis.
The characteristic of glioma is the immunosuppressive tumor microenvironment (TME); immunosuppression is caused owing to the evolution of T cell tolerance against tumor-selective antigens. OVs have been reported to significantly increase immune cell infiltration and activate inflammation of the TME. Genetically modified OVs are engineered to destroy the TME. In addition, Oncolytic Virotherapy has to clear a lot of hurdles like Blood Brain Barrier (BBB) existing between CNS and vascular compartments, TME, and high molecular variability of tumor cells [4]. They attack the non-injected malignant visceral mass of cells around the tumor (Table 1) [5].
The cause for such prevalence includes ionizing radiation, air pollution, and an aging population. Glioblastoma multiforme (GBM) is the most prevalent glioma around the globe, occurring in 77%-81% of malignant tumors associated with the Central Nervous System (CNS) [6]. As per World Health Organisation (WHO), it is denoted as a diffuse astrocytic and oligodendroglial tumor belonging to grade IV [7]. The median survival is approximately 14.6 months [6]. As per the literature survey, rates of CNS and brain cancers in Eastern and Southern Europe and South America were rising, while the rates were only reported to fall in Japan in 2017 [7]. The possible causes for this rising prevalence are still yet unknown, and mere causal factors can be hypothesized.
Table 1. Oncolytic viruses and their immunological responses in different tumors and their phases [5]
| Virus | Tumor | Immunological response |
|---|---|---|
| Adenovirus | Pancreatic cancer Solid tumors | Enhanced long-term survival and T-cell response to tumors |
| Herpes simplex virus | Glioma Melanoma | 34% regression of uninjected non-visceral mass and 15% visceral tumors |
| Vaccinia | Colorectal cancer Head and neck carcinoma | Interleukin 6 and TNF-α peak at 3 hours and later time |
2. Challenges
The challenges OVs face are neutralization by complement factors and antibodies immune cell responses. OVs have to cross the BBB to reach the tumor site, which is a significant challenge for the success of this therapy (See figure 1). The architecture of BBB in the CNS-regulating passage is very stringent, which disallows the entry of specific molecules across them [6]. Exceptions exist, as some viruses can cross the BBB. OVs are administered by injecting intratumorally or intravenously, depending on the accessibility of the tumor’s location [7].
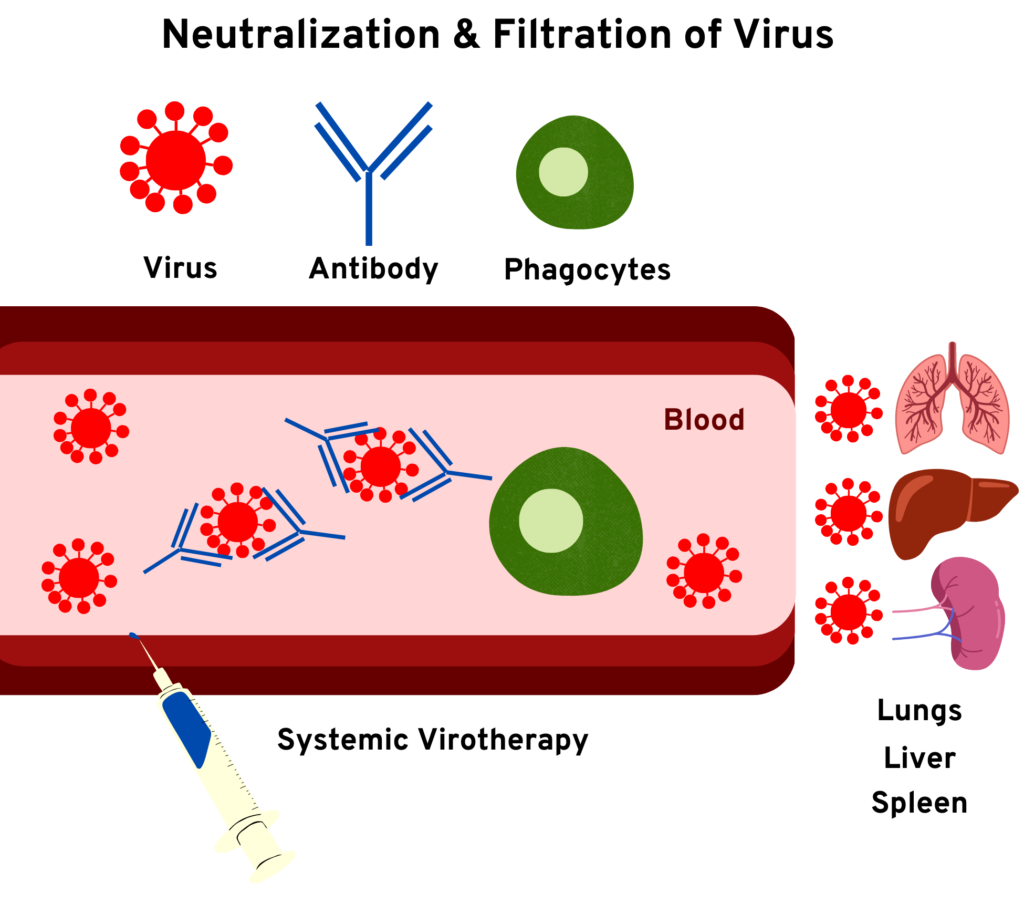
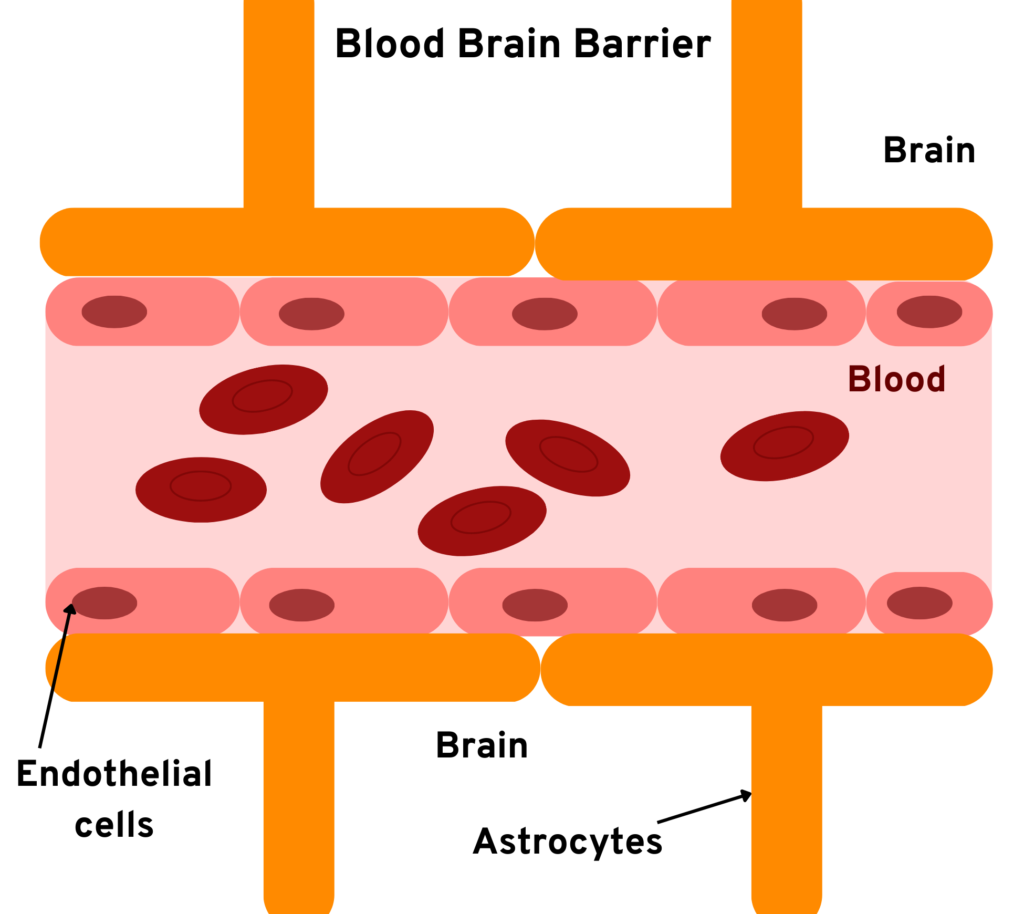
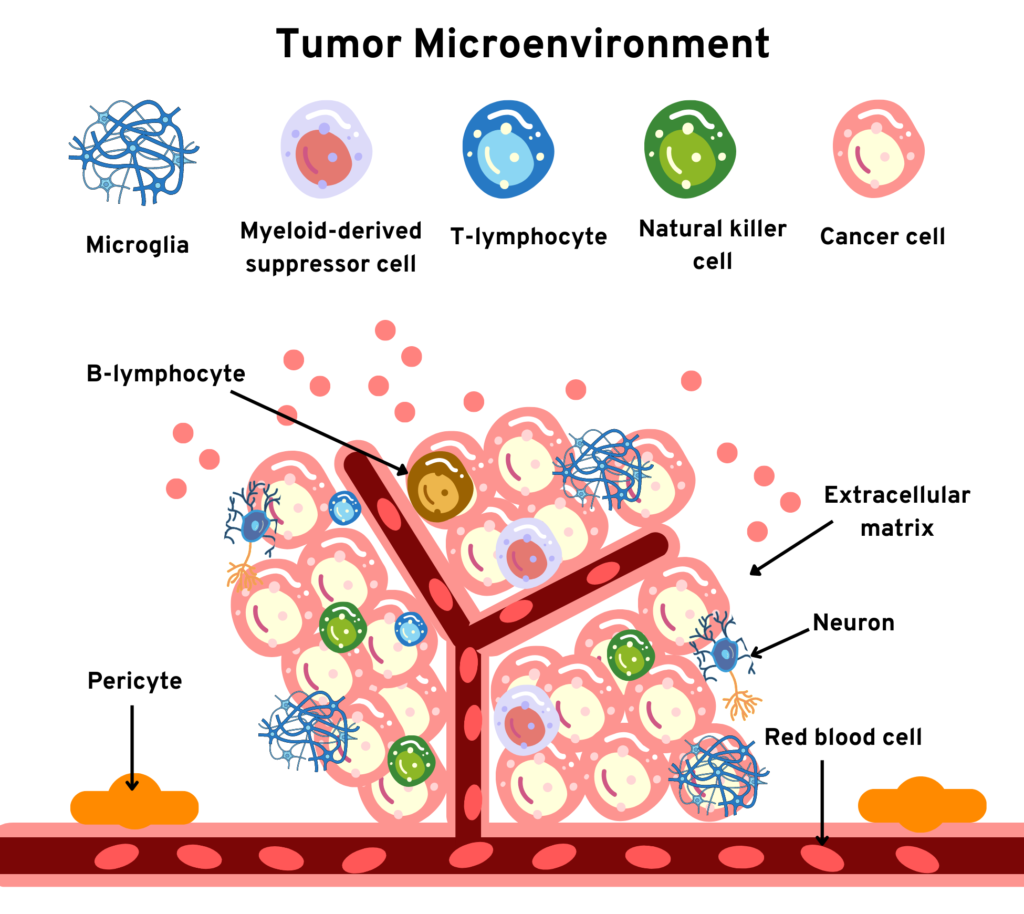
Figure 1. Obstacles in the administration of Oncolytic Virotherapy (schematic representation)
To summarise, Oncolytic Virotherapy is a potent therapy that has the potential to cure cancer. There are also limited OVs available for the treatment of patients. Another challenge is that the virus might get wiped out by the body’s natural immune response. It might also get hindered by the BBB and never reach the tumor site [8]. This virotherapy can combine with existing therapies to give synergistic effects [9]. The future scope of this therapy will be an organic combination of gene modifications and constructions of virus vectors. Its efficiency also depends on the individual’s physical environment, which is unique to everyone.
3. Conclusion
Further research should also be carried out on the biosafety of virotherapy, especially for low immunity people [10]. Virotherapy extends the survival rate of patients and helps them lead healthy lives in the future. Virotherapy, with substantial research, may emerge as a new therapy to cure cancer.
References
[1] Y.R. Suryawanshi, A.J. Schulze, Oncolytic viruses for malignant glioma: on the verge of success? Viruses. 13 (2021) 1294.
[2] M.E. Davis, Glioblastoma: overview of disease and treatment, Clin. J. Oncol. Nurs. 20 (2016) S2.
[3] M. Zheng, J. Huang, A. Tong, H. Yang, Oncolytic viruses for cancer therapy: barriers and recent advances, Mol. Ther.-Oncolytics. 15 (2019) 234–247.
[4] R.K. Upadhyay, Drug delivery systems, CNS protection, and the blood brain barrier, BioMed Res. Int. 2014 (2014).
[5] N. Grech, T. Dalli, S. Mizzi, L. Meilak, N. Calleja, A. Zrinzo, Rising incidence of glioblastoma multiforme in a well-defined population, Cureus. 12 (2020).
[6] Q.T. Ostrom, H. Gittleman, P. Farah, A. Ondracek, Y. Chen, Y. Wolinsky, N.E. Stroup, C. Kruchko, J.S. Barnholtz-Sloan, CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010, Neuro-Oncol. 15 (2013) ii1–ii56.
[7] D.N. Louis, A. Perry, G. Reifenberger, A. Von Deimling, D. Figarella-Branger, W.K. Cavenee, H. Ohgaki, O.D. Wiestler, P. Kleihues, D.W. Ellison, The 2016 World Health Organization classification of tumors of the central nervous system: a summary, Acta Neuropathol. (Berl.). 131 (2016) 803–820.
[8] R. Stupp, W.P. Mason, M.J. Van Den Bent, M. Weller, B. Fisher, M.J. Taphoorn, K. Belanger, A.A. Brandes, C. Marosi, U. Bogdahn, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma, N. Engl. J. Med. 352 (2005) 987–996.
[9] A. Filley, M. Dey, Immune system, friend or foe of oncolytic virotherapy? Front Oncol 7: 106, (2017).
[10] A. Philips, D.L. Henshaw, G. Lamburn, M.J. O’Carroll, Brain tumours: rise in glioblastoma multiforme incidence in England 1995–2015 suggests an adverse environmental or lifestyle factor, J. Environ. Public Health. 2018 (2018).
